How Trading Partners Can Prepare for DSCSA
As the DSCSA interoperability deadline approaches, we at NABP remain focused on critical Drug Supply Chain Security Act (DSCSA) infrastructure and committed to helping align all sectors of the supply chain that support and provide safe medication to patients in the United States. This is the fourth blog of a series that explores steps we […]
Read More ›How the Industry Can Enable a Realistic Adoption to DSCSA Compliance
As the DSCSA interoperability deadline approaches, we at NABP remain focused on critical Drug Supply Chain Security Act (DSCSA) infrastructure and committed to helping align all sectors of the supply chain that support and provide safe medication to patients in the United States. This is the third blog of a series that explores steps we […]
Read More ›NABP Supports a Flexible Approach to DSCSA Digital Credentials
As the DSCSA interoperability deadline approaches, we at NABP remain focused on critical Drug Supply Chain Security Act (DSCSA) infrastructure and committed to helping align all sectors of the supply chain that support and provide safe medication to patients in the United States. This is the second blog of a series that explores steps we […]
Read More ›The 4 Biggest Hurdles to DSCSA Compliance
As the DSCSA interoperability deadline approaches, we at NABP remain focused on critical Drug Supply Chain Security Act (DSCSA) infrastructure and committed to helping align all sectors of the supply chain that support and provide safe medication to patients in the United States. This is the first blog of a series that explores steps we […]
Read More ›Task Force Update: NABP Initiatives Tackle Medication Errors, New Pharmacy Practice Models
January is a busy time for NABP. We’re preparing for the 119th Annual Meeting in May and mapping out the upcoming year’s goals and initiatives. In addition, we’re continuing to provide guidance and support on the issues that protect patient safety and are of high importance to the boards of pharmacy. These are projects that […]
Read More ›New Bill for Online Platform Accountability Looks Beyond Section 230
As has been reported extensively over the past year, social media companies have come under scrutiny both at the national and international levels for not doing enough to prevent their platforms from being used to facilitate illegal drug sales. Despite reports that many popular platforms have stepped up efforts to police content – both TikTok […]
Read More ›How Pharmacies Can Prep Now for the 2023 DSCSA Requirements
When Food and Drug Administration (FDA) enacted the Drug Supply Chain Security Act (DSCSA) in 2013, it set a 10-year timeline for full implementation. Well, believe it or not, we are just a little more than a year away from that November 2023 deadline. A significant portion of this last milestone requires the entire supply […]
Read More ›Regulatory Agencies Worldwide Are Partnering to Combat Illegal Online Pharmacies
At the end of July, INTERPOL announced the execution of Operation Pangea XV, an annual week of coordinated law enforcement action to combat the illegal sale of medicines and medical products online. From June 23 to June 30, 2022, 94 member countries spanning every continent issued over 200 search warrants and opened over 600 investigations. […]
Read More ›Update on DRUGS Act: Bipartisan Bill Seeks to Shut Down Illegal Online Pharmacies
For over two decades, NABP has raised concerns over the vast number of illegal online pharmacies that put Americans’ health at risk by selling medications without a prescription, operating without a license, and peddling substandard, falsified, or counterfeit prescription drugs, including illicit opioids, to patients. Government agencies, including Food and Drug Administration (FDA), Drug Enforcement Administration (DEA), […]
Read More ›NABP Pilot Brings Trading Partners, Regulators Together for Public Health Protection
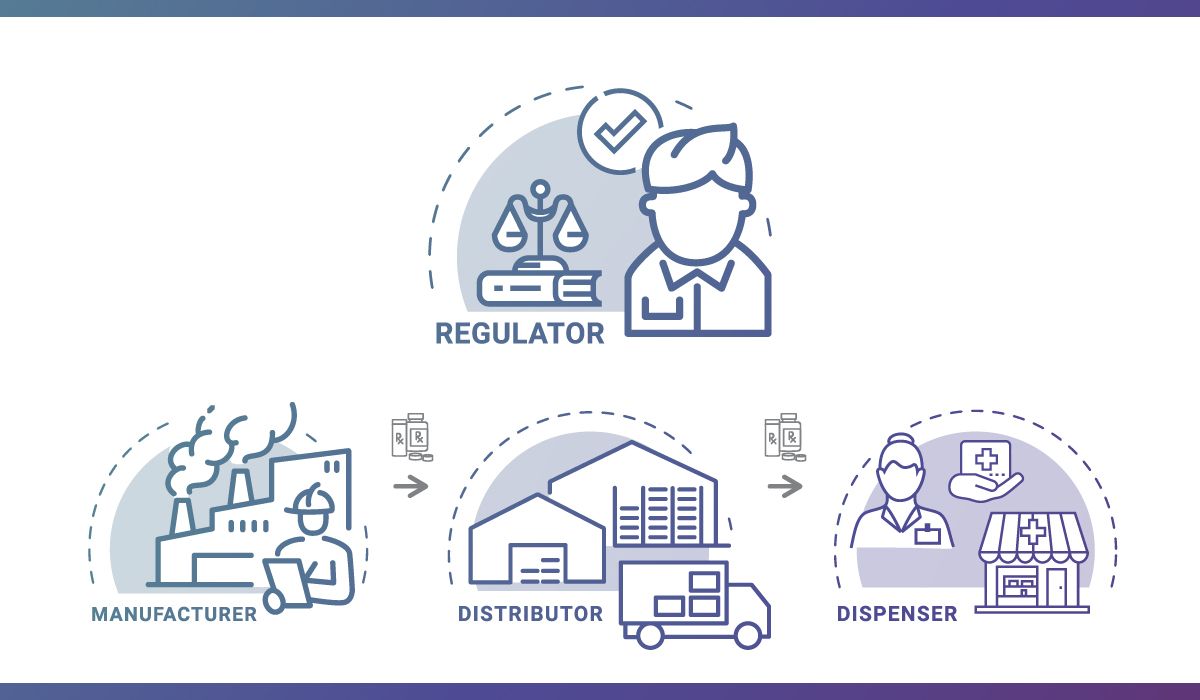
By November 27, 2023, the Food and Drug Administration’s (FDA) Drug Supply Chain Security Act (DSCSA) will be fully implemented. We’ve been working with the boards of pharmacy, other regulators, and trading partners to prepare for the requirements included in Title II of the Act, which calls for product tracing at the package level. Our […]
Read More ›