This column was prepared by the Institute for Safe Medication Practices (ISMP), an ECRI affiliate.
The Institute for Safe Medication Practices (ISMP) has recently received multiple reports of different manufacturer bottles with similar appearances that have contributed to errors. In one case, a mix-up occurred between prasugrel 10 mg tablets and flecainide 100 mg tablets, both manufactured by Amneal Pharmaceuticals. A patient had undergone a percutaneous coronary intervention in the hospital’s catheterization laboratory and had a stent placed. Following the procedure, the patient was prescribed prasugrel, an antiplatelet agent, and directed to take 10 mg daily. The prescriber wrote the prescription for a 90-day supply. However, the pharmacy dispensed a mix of prasugrel and flecainide, an antiarrhythmic agent, to the patient.
Amneal manufactures prasugrel 10 mg tablets in bottles containing 30 tablets, and product labeling requires the pharmacy to dispense the medication in the original manufacturer’s container. As a result, to fill a 90-day supply, the pharmacy must dispense three unopened bottles. However, this pharmacy also stocks 100-count bottles of flecainide 100 mg tablets from Amneal, which look nearly identical to the prasugrel bottles. Both bottles are the same size, are white with white lids, and the same colors and layouts are used on the container labels. Due to the look-alike packaging, staff had inadvertently shelved the flecainide bottles with the prasugrel bottles.
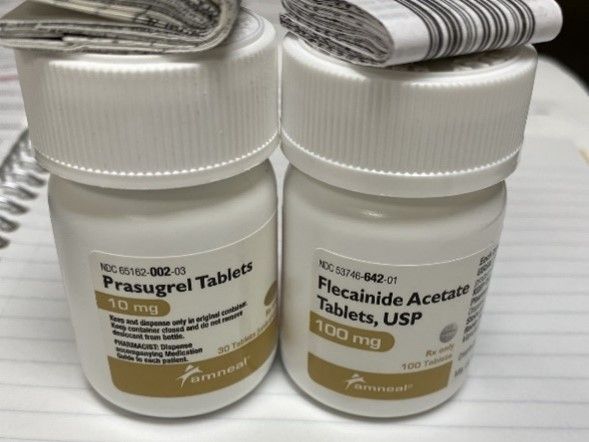
Figure 1. Bottles of prasugrel 10 mg tablets (left) look very similar to bottles of flecainide 100 mg tablets (right), both marketed by Amneal. Flecainide bottles were inadvertently stored with the prasugrel bottles and subsequently dispensed instead of prasugrel.
When filling the prescription, a pharmacy technician accidentally grabbed one bottle of flecainide and only two bottles of prasugrel. They then affixed pharmacy prescription labels for prasugrel to each bottle. During product verification, the pharmacist scanned the barcode of only one bottle, which was all that was required by the pharmacy computer system. The bottle they scanned happened to be a prasugrel bottle, so they did not receive an error message and then did not recognize that one of the bottles contained flecainide and not prasugrel. At home, the patient opened the bottle of flecainide first and took the wrong medication for a month. They did not realize the error until they opened a bottle containing prasugrel.
To help prevent errors with look-alike packaging, explore purchasing one medication from each of these pairs from a different manufacturer. If you currently have these products, consider separating them; make sure staff members are aware that they have been separated and know where to locate the medications. The pharmacy should employ processes and technology that can intercept product selection errors. For example, pharmacies should utilize barcode scanning during production and scan each bottle used to fill a prescription, including each manufacturer bottle that may be dispensed to a patient. The pharmacy computer system should also require the pharmacist to scan each bottle dispensed during product verification. Avoid obscuring critical information (eg, drug name, dosage strength, preparation instructions) on the manufacturer label, whether this is marking the containers with an “x” or affixing auxiliary labels, price stickers, or other labels. At the point of sale, open the bag and have the patient check what has been dispensed to make sure it is correct.