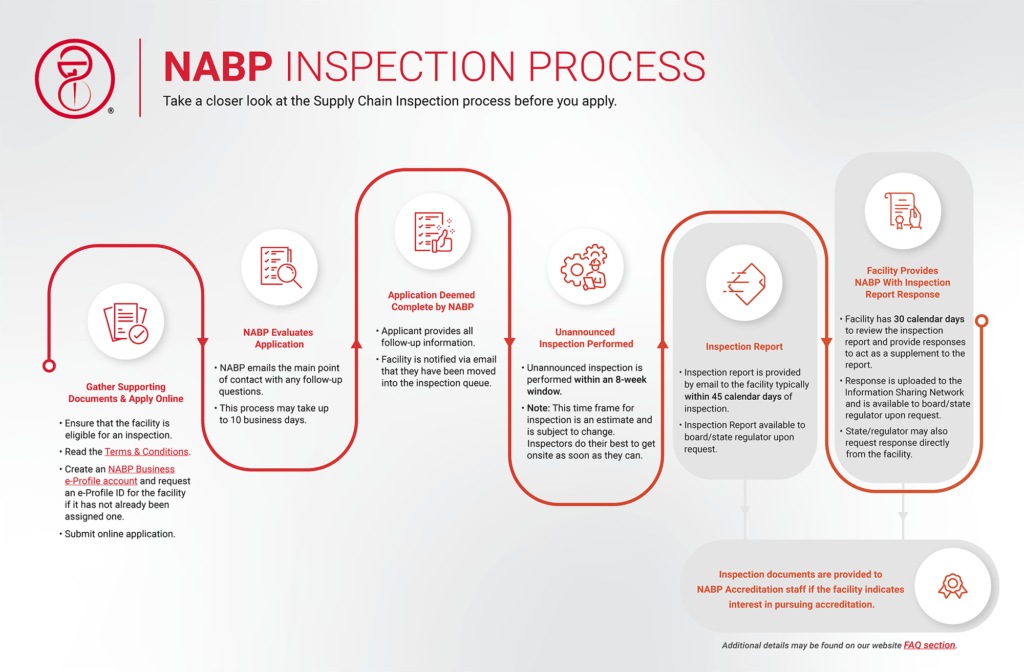
What can we expect during the Supply Chain Inspection?
After the application and payment has been submitted, your facility may be inspected at any time. Your facility is expected to be familiar with and be operating under all applicable regulations. The inspection is considered to be a “snapshot in time”, which means the report will reflect the observations and current conditions at the time of the inspection.
As this is a general inspection, some items reviewed may not be required by the resident state. NABP does not make a final determination or draw a conclusion that your facility has “passed” the inspection or is “compliant” for a specific state regulation. It is up to each individual state to review the inspection report and determine whether the observations documented in the Supply Chain Inspection meet state (and/or federal) requirements.
It is highly encouraged to have someone available during the onsite inspection who can pull records.
Supply Chain Inspection
This inspection covers informational and process questions in the following areas:
- Background
- Additional Record Requests/Reviews
- DSCSA (Section 581 and 582 of the FD&C Act)
- Legitimate OTC Medical Device Determination
- Laws/Regulations
- Reporting and Notifications
- Facility Licenses/Permits/Registrations
- Personnel
- Source/Vendor Verification
- Customer Verification
- Facility
- Security
- In-Bound Drug/Device Inventory and Receiving
- Temperature and Humidity
- Quarantine
- Inventory
- Order Fulfillment and Shipping
- Controlled Substances Recordkeeping and Record Retention
Some areas of the inspection may not be applicable to your facility.
Outsourcing Facility
This module covers informational and process questions in the following areas:
- FDA Inspections
- Recalls
- Quality Program
- Compounding