Apply for VPP Inspection
How to Get Started
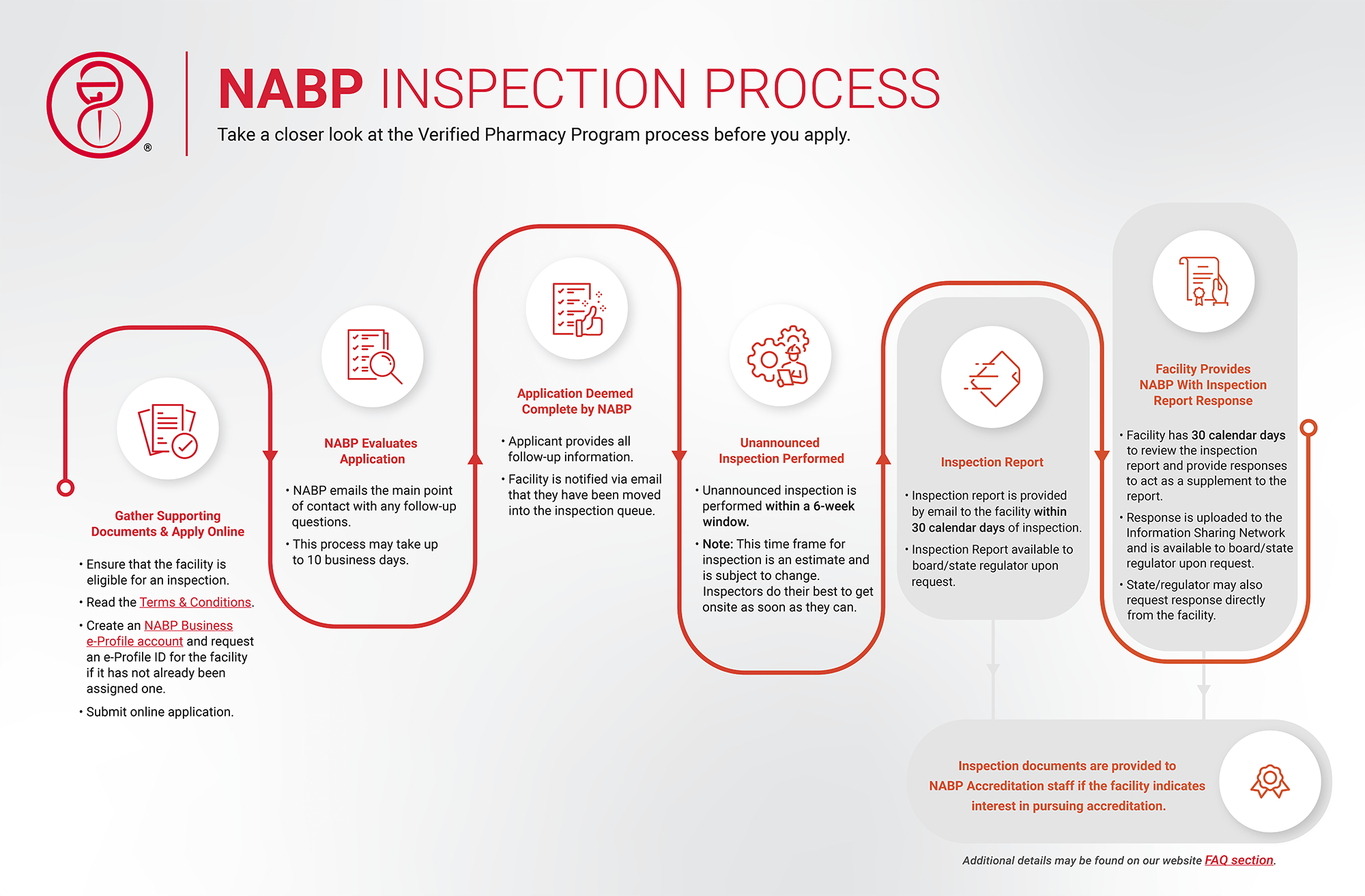
First, apply for a VPP Inspection. Once your application, required documentation, and specified fees have been received and you have agreed to the terms and conditions:
- NABP conducts an unannounced on-site inspection of all your pharmacy’s activities.
- All business e-Profile information is accessible to the state boards of pharmacy to view.
- The board staff receives alerts when new documents are available on the business e-Profile for pharmacies that are licensed and or seeking licensure.
- State boards will be contacted if an inspection will be completed in their state and resident state inspectors may observe.
Apply for Inspection
Get started on your path to an inspection by following the steps below:
- Confirm your eligibility.
- Create a business e-Profile account:
- Review the information needed and basic instructions when creating your business e-Profile.
- Use this guide to request access to additional Business e-Profile accounts
,after creating your initial e-Profile. - Once your request has been submitted, it may take up to 3 business days for processing. You can track the status of your request in e-Profile.
- Gather supporting documents for the application.
- Submit the application for one or multiple locations.
- Read the Application Basics document for instructions on submitting your application.
Interested in pricing?
Log in to your business e-Profile account and visit the Instructions and FAQs section to access pricing. Don’t have an account? Create your free business e-Profile account today.
Review the policy information prior to starting the VPP application.