Preparing for an Inspection or Accreditation Survey – Part 2
This blog post is continued from Preparing for an Inspection or Accreditation Survey – Part 1. Read part 1 for more information on preparing for an on-site visit by a third-party organization or regulatory agency for an inspection, survey, or review of your operations. Conduct Mock On-site Visits One of the best ways to […]
Read More ›Preparing for an Inspection or Accreditation Survey – Part 1
Whether you are opening a new facility, seeking an accreditation, or operating business as usual, at some point, you will encounter a third-party organization or regulatory agency. These groups will come on-site for a visit to inspect, survey, or review your operations. Preparation for this on-site visit is the key to a successful outcome. Steps […]
Read More ›What Trading Partners Need to Know Before the DSCSA Deadline
Full implementation of the Drug Supply Chain Security Act (DSCSA) was to begin on November 27, 2023. However, on August 25, 2023, US Food & Drug Administration (FDA) issued what it refers to as a “Stabilization Policy,” — a one-year period of non-enforcement for certain DSCSA requirements. In our view, FDA expects drug supply chain […]
Read More ›Update on DRUGS Act: Bipartisan Bill Seeks to Shut Down Illegal Online Pharmacies
For over two decades, NABP has raised concerns over the vast number of illegal online pharmacies that put Americans’ health at risk by selling medications without a prescription, operating without a license, and peddling substandard, falsified, or counterfeit prescription drugs, including illicit opioids, to patients. Government agencies, including Food and Drug Administration (FDA), Drug Enforcement Administration (DEA), […]
Read More ›NABP Pilot Brings Trading Partners, Regulators Together for Public Health Protection
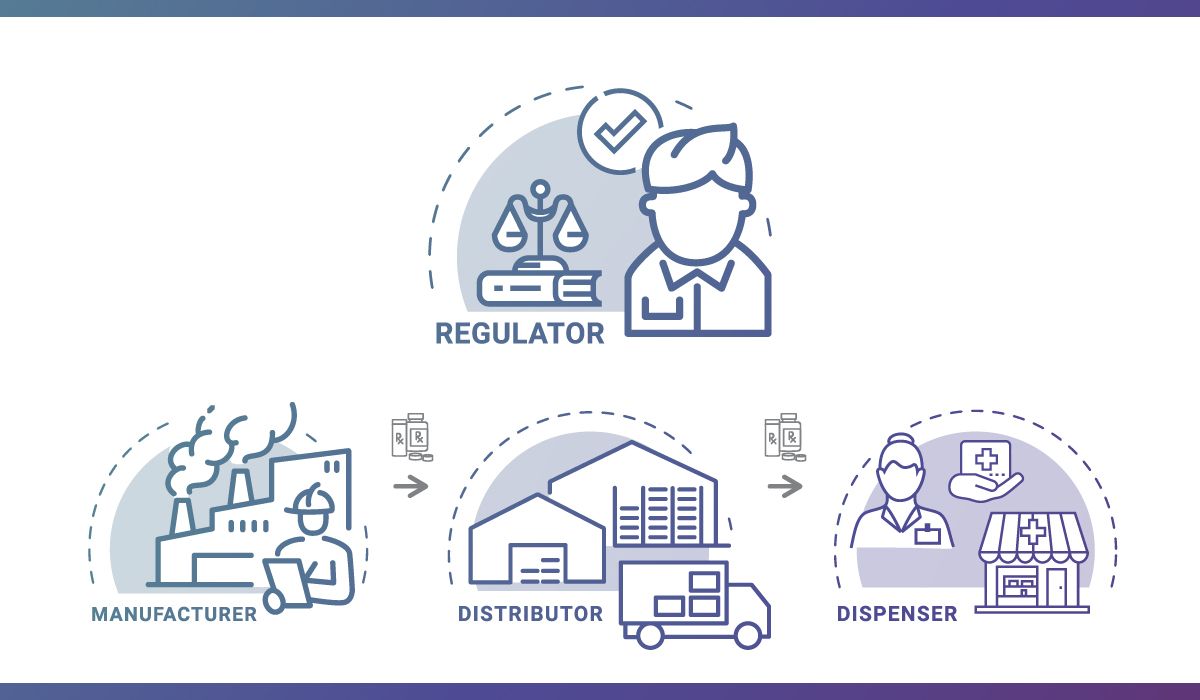
By November 27, 2023, the Food and Drug Administration’s (FDA) Drug Supply Chain Security Act (DSCSA) will be fully implemented. We’ve been working with the boards of pharmacy, other regulators, and trading partners to prepare for the requirements included in Title II of the Act, which calls for product tracing at the package level. Our […]
Read More ›Bipartisan, Bicameral Bill Seeks to Address Illegal Drug Sales on the Dark Web
Congress has long had an interest in stemming the online sale of illegal opioids but investigating and identifying criminals who traffic these drugs on the dark web is incredibly challenging for law enforcement agencies. Enter the Dark Web Interdiction Act of 2022 (HR 7300, S 3782), a new bipartisan bill that seeks to improve the […]
Read More ›Congress Prepares for the Next Phase of the Pandemic and Transformations in the Telehealth Industry
With the release of the White House’s next phase National COVID-19 Preparedness Plan and looming deadlines to fund the federal government, including replenishing aid for pandemic relief, it has been an eventful month on Capitol Hill for the health care industry. In the most recent iteration of President Joseph R. Biden’s pandemic preparedness plan, the White House […]
Read More ›DEA Seeks Public Input as it Explores Regulating Telepharmacy
The coronavirus disease 2019 pandemic prompted state and federal government to shift guidance and relax policies on a wide range of telehealth services, which resulted in a boom in remote offerings for patients. However, long before this rapid transformation in telehealth, telepharmacy, an increasingly popular form of remote pharmacy care, has operated under a patchwork of state laws with […]
Read More ›How e-Prescribing Trends Impacted the Opioid Epidemic

Before DEA published an interim final rule in 2010, which gave practitioners the option to write prescriptions for controlled substances (CS) electronically, e-prescribing for CS was prohibited in many states. Now, 11 years later, the regulatory and technological landscape has shifted significantly. As part of the larger effort to curb the opioid crisis, many states […]
Read More ›Congress Holds Registries and Registrars Accountable for Rogue Online Pharmacies

As has been reported extensively over the last several months, policymakers on both sides of the aisle have been unwavering in their pursuit to hold tech companies and social media platforms accountable for building a safer internet. Until recently, their focus has predominantly been on online platforms that serve as publishers of third-party content, such […]
Read More ›